Abstract
Introduction: Lenalidomide, bortezomib, and dexamethasone is a standard of care in the treatment of fit multiple myeloma patients due to high efficacy, with ORR exceeding 90% in the first-line setting (Richardson et al. 2010). The initial RVD regimen utilized a 21-day cycle with bortezomib administered IV 1.3 mg/m2 days 1, 4, 8, and 11; lenalidomide 25 mg administered days 1-14; and dexamethasone administered 160 to 320 mg per cycle. However, toxicity may be treatment-limiting, with bortezomib-induced neuropathy affecting up to 80% of patients. Modifications to RVD to optimize tolerance include reducing the bortezomib dose to a weekly schedule (1.6 mg/m2 IV or 1.3 mg/m2SC) and extending the cycle to 28 or 35 days (Broijl et al. 2016; O'Donnell et al. 2014). Notably, those regimens were studied in transplant-ineligible or relapsed/refractory patients. Based on regimens like CyBorD (which utilize weekly SC administration of bortezomib, are well-tolerated and are efficacious), we modified the standard 21-day RVD regimen to include 3 doses of weekly bortezomib in an attempt to preserve efficacy while minimizing toxicity. We present a retrospective analysis of both fit and transplant-ineligible patients treated at our institution using "Louisville RVD."
Methods: We identified the charts of patients who received RVD over a 21-day cycle at the following starting doses: lenalidomide 25 mg orally days 1-14; bortezomib 1.5 mg/m2 SC days 1, 8, and 15; and dexamethasone 40 mg days 1, 8, and 15. Autologous stem cell harvest took place after 4 cycles of lenalidomide-based therapy when feasible, in appropriate candidates. We noted demographic factors, ISS stage, performance status, cytogenetic risk stratification, line of therapy (initial, later-line, and consolidation), history of autologous transplant, degree of response (PD, SD, PR, VGPR, and CR based on International Myeloma Working Group criteria), EFS, OS, and toxicities.
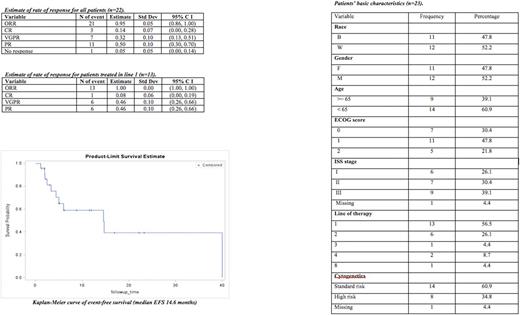
Results: We identified 23 patients who received Louisville RVD between 2009 and 2017. Median age was 59 years (range 37-77) at treatment start; 9 (39%) were 65 or older; 12 (52%) were male; 11 (48%) were African-American; ISS stages were I in 6 (26%), II in 7 (30%), III in 9 (39%), and missing in 1 patient; ECOG status was 0 in 7 (30%), 1 in 11 (48%), and 2 in 5 (21%). Line of therapy was 1 in 13 (56%) and 2 or later in 10 (44%); RVD was used as post-transplant consolidation in 4 (14%). Line of therapy ranged from 1 to 8. 16 patients (57%) were treated with intent to transplant. The median number of cycles given was 4 (range 1-64). Dose reductions and discontinuation occurred in 5 (22%) patients and 1 (4%) patient after 1 cycle, respectively. Response was assessable in 22 patients. 21 (95%) of patients achieved PR or better; 3 patients (14%) achieved CR, 7 (32%) VGPR; and 11 (50%) PR. OS and EFS at 1 year were 90% and 60%, respectively; median duration of follow-up was 15.5 months (range 1.2-95.1); follow-up time was limited for a significant fraction of our patients. There was no association of relapse with ISS stage (p=0.357), ECOG score (p=0.088), or cytogenetic risk (p=1.00). Treatment-related toxicities were reported for 11 patients. Treatment-emergent neuropathy was seen in 5 patients (22%); 4 patients experienced grade 1 neuropathy (17%), and 1 patient experienced grade 3 neuropathy (4%). 1 patient underwent dose-reduction of bortezomib to 1.3 mg/m2 after 18 cycles. 6 patients had treatment related cytopenias (26%), of which 1 (4%) was grade 3.
Conclusions: Retrospective analysis of 21-day "Louisville RVD" with weekly subcutaneous bortezomib demonstrates efficacy comparable to previously published RVD regimens. Toxicity, especially peripheral neuropathy, favors the weekly SC administration schedule. Dose reductions were uncommon and treatment discontinuations were rare. Patients may ultimately achieve superior bortezomib dosing due to rarity of dose reductions. Our cohort was well-balanced with regard to ethnicity, gender, age, and performance status. Treatment was efficacious and well-tolerated in both transplant and non-transplant candidates.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal